long-term care best practices, industry updates, and more
The LTC Innovation Blog
Subscribe

August 22, 2022
How Can Clearinghouse Partnerships Support Medical Billing in the Pharmacy?
What does it take to get a paid claim for a prescription? Ask any pharmacy billing specialist and they can tell you exactly how to do it. Pharmacy billing claims use the NCPDP Telecommunications Standard D.0 claim format, a code system that communicates prescription claim data to third party payers. Submitted claims are adjudicated almost immediately, informing the pharmacy whether the claim is approved or denied. Medical claims are distinctly different and more complex. Medical claims require a larger data set and are not adjudicated immediately, sometimes taking weeks to receive an approval or rejection. Creating a claim to a medical payer cannot be directly accomplished using a standard pharmacy operating system. To submit medical claims today, pharmacies must either submit a paper CMS-1500 claim form, or employ an entity known as a clearinghouse to translate the claim from NCPDP D.0 to ANSI-X12 837, the standard format used to send health care claims electronically.
Read More
June 29, 2022
|
FrameworkRxP
Why Add FrameworkRxP to Your LTC Pharmacy Software Tech Stack?
Monthly medication regimen reviews (MRRs) are standard practice in long-term care (LTC). Consulting pharmacists conduct MRRs to ensure the right prescriptions are being dispensed in the right dosage and formula for each patient—not to mention being properly monitored over time. For skilled nursing facilities (SNFs) in particular, this process is getting more and more complex with an increasing number of regulations to follow around the appropriate use of medications. These constraints make conducting accurate, efficient MRRs very challenging—at least without the right LTC pharmacy software.
Read More

June 16, 2022
|
Regulatory
Scammers Are Targeting Pharmacies – Learn How To Identify Them
Across the country, many states are reporting that scammers are targeting pharmacies, pretending to be Board of Pharmacy (BOP) inspectors, DEA agents, the FBI, or other false identities.
Read More

June 3, 2022
Should Congress Pass the BLOCKING Act of 2021?
In April of 2021, a bill was introduced to the House of Representatives called the Bringing Low-cost Options and Competition while Keeping Incentives for New Generics Act of 2021 (or simply, the BLOCKING Act). This bill, if passed into law, would remove the 180-day exclusivity period for new generic drugs. While the intent is to expedite more generic drug entries to the market, some experts fear it may have the opposite effect by disincentivizing generic manufacturers to enter the space in the first place. Currently, the Food and Drug Administration (FDA) awards 180 days of exclusivity on the market to the first applicant to file a qualifying application for market approval of a generic drug. Generally, this exclusivity period begins upon the first applicant's commercial marketing of the drug. The bill authorizes the FDA to approve a subsequent generic drug application prior to a first applicant's first date of commercial marketing if (1) the subsequent application is ready for full approval, (2) a first applicant's application has been pending for at least 30 months, and (3) the approval of a first applicant's application is not precluded by patent infringement claims asserted against that first applicant. What do you think? Would the BLOCKING Act be good for pharmacies and patients? Would it lead to more generics on the market sooner? Or, would it discourage manufacturers by removing the incentive of market exclusivity?
Read More
May 23, 2022
|
FrameworkLTC,
Clinical
Understanding Biosimilars
Summary: This article explores the imminent release of a feature within FrameworkLTC aiding pharmacies in making informed decisions on biosimilar substitutions, accompanied by an overview of biologic drugs, their classifications, and the regulatory landscape surrounding their substitution. Table of Contents
Read More
May 17, 2022
|
FrameworkECM,
frameworkvision
How Secure Messaging Works in LTC Pharmacy Software
Seamless communication among pharmacies, facilities, and prescribers is essential in long-term care (LTC). When all key players are able to communicate, collaborate, and coordinate care efficiently and effectively, everyone benefits; however, outdated forms of communication have led to endless games of phone tag and fax headaches that impede effective coordination of care.
Read More
May 16, 2022
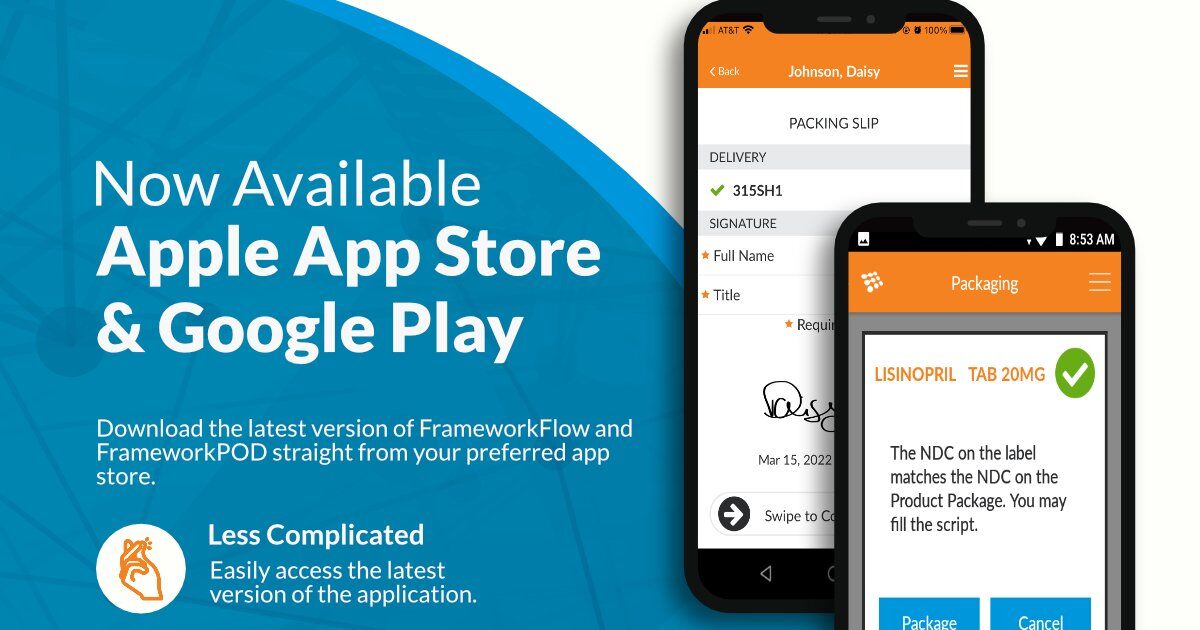
FrameworkFlow & FrameworkPOD Now Available on Apple and Google Play.
SoftWriters is excited to announce that FrameworkFlow and FrameworkPOD are now available on both the Apple App Store and Google Play. Both apps will continue to deliver the same high-quality experience you have come to love but will now be easier to update and access.
Read More

May 5, 2022
What Are the Benefits of Pharmacy Barcode Scanners?
Summary: This article emphasizes the transformative impact of barcode scanner technology in long-term care (LTC) pharmacies.
Read More